 Research Interests:
Research Interests:
- Redox processes
- Cellular detoxification
- Enzymology/Protein chemistry
- X-ray crystallography
- Structural biology
Research Overview
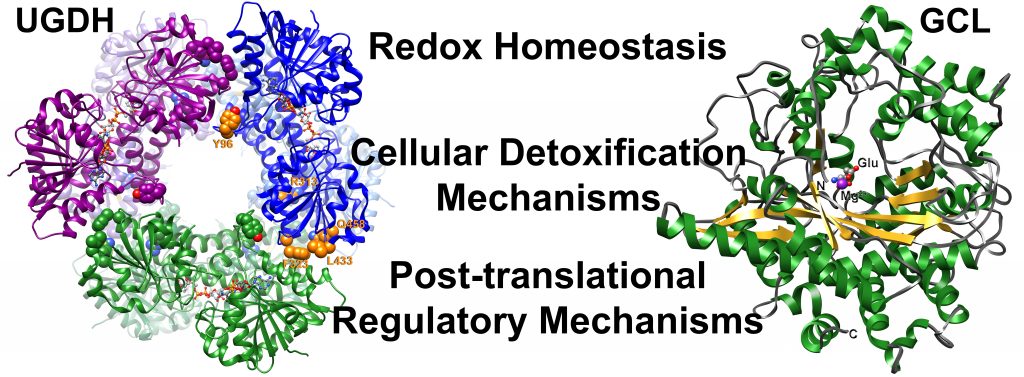
My research program is focused on cellular detoxification pathways. Glutathione, an abundant low molecular weight thiol, is largely responsible for the tightly controlled maintenance of intracellular reduction-oxidation status essential for normal cellular function. Despite its ubiquitous distribution and critical functions in mammalian systems, numerous aspects of glutathione homeostasis remain unresolved. My laboratory uses a variety of biophysical techniques, including protein crystallography, enzymology, and protein chemistry, to examine the mechanisms of the enzymes responsible for maintaining reduced glutathione reservoirs. We have also recently developed eukaryotic model systems to validate our results in relevant contexts. Our work has provided a structural framework to examine regulation of glutathione metabolism at a molecular level, with the ultimate goal of controlling flux through the glutathione pathway.
A major focus of the lab is examining how small molecule metabolites involved in cellular detoxification pathways, are directed to a given metabolic fate. In collaboration with the Simpson laboratory, we have been studying human UDP-glucose dehydrogenase. The product of this enzyme, UDP-glucuronate, has similar detoxification functions as glutathione. We are attempting to determine the regulatory mechanisms that dictate partitioning of UDP-glucuronate to one of three fates: hyaluronan production, proteoglycan biosynthesis, and detoxification by UDP-glucuronosyltransferases.
